Cholera
Cholera: origin and transmission
Cholera is a toxic contagious enteric epidemic infection caused by Vibrio cholerae, serotype O1 or O139. It leads to acute watery diarrhea which can rapidly evolve to dehydration and death if left untreated. Most patients will recover with oral rehydration therapy; however, some patients will require rapid intravenous rehydration. The death rate can be less than 1% of cases with adequate treatment; however, this can be as high as 50% if cases are left untreated.
Cholera is transmitted via food or contaminated water and can be prevented by ensuring clean water, good sanitation, and effective hygiene. Cholera can also be controlled through vaccination. The cholera vaccine is an oral vaccine consisting of 2 doses that are administered two weeks apart.
Cholera in endemic in south-east Asia and causes outbreaks in Africa and the Americas. An increased risk of cholera has been noted in parallel with the ever-increasing size of vulnerable populations living in unsanitary conditions, in slums were basic sanitation infrastructure is lacking, or due to a breakdown of the infrastructure as a result of war or a natural disaster. Cholera affects n estimated 1.3 to 5 million people worldwide each year and causes 21,000–143,000 deaths a year.
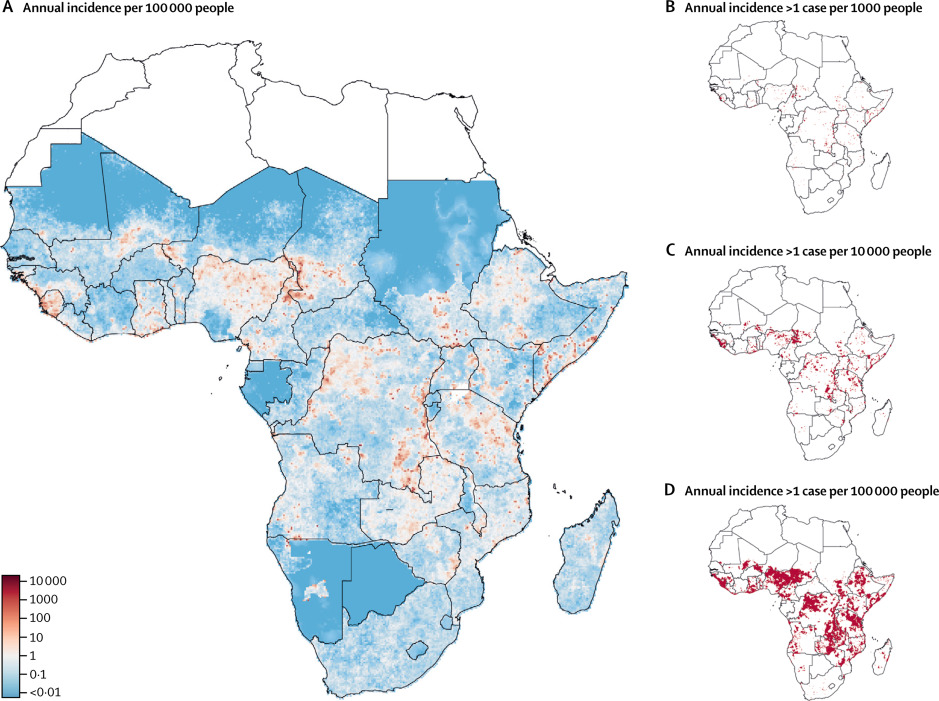
Mapping the burden of cholera
Concentration the effort in the most affected places
Epicentre and the Johns Hopkins University have mapped the areas where cholera is most prevalent in Africa using modeling techniques1 . Results indicate that a concentrated effort in just 5% of all districts in Africa could reduce by half the disease burden in the continent. These findings are the linchpin of the Cholera Global Roadmap to 2030, which outlines the strategy to eliminate cholera transmission as well as providing the key rational for cholera control investment case.
Concentrating on the important strains
A complementary collaboration with Institut Pasteur, in which the whole genome sequencing of more than 1000 V. cholerae isolates has been analyzed, has shown that 7th pandemic - the current pandemic which started in South Asia in 1961 and reached Africa in 1971 and the Americas in 1991 - strains have been introduced in Africa several times in the last 50 years from Southern-Asia. The circulation of strains can be monitored by observing the emergence of hotspots in neighbouring countries in Africa. This makes it possible to anticipate the areas where such hotspots may appear and focus cholera control activities there. This helps prevent the reintroduction of new viruses.
Understanding cholera outbreaks
Better estimates despite underreporting of cases
Retrospective morbidity and mortality studies conducted by Epicentre in Haiti demonstrated that the true number of cholera cases was much higher than recorded because remote villages are beyond the reach of health services3.
Epicentre provides field epidemiology support to Médecins Sans Frontières (MSF) to describe cholera outbreaks in terms of time, place and person. Epicentre has developed as well models to better understand the cholera epidemics spread, as it has been recently the case in Yemen4. In addition, Epicentre has shown using spatial epidemiological methods that cholera spreads in clusters.
Exploring suitable intervention strategies
Epicentre is exploring a better intervention strategy referred to as case-area target interventions, which can include WASH interventions, ring vaccination as well as distribution of antibiotics to household members. This strategy consists of containing the first foci of infection. The first results observed in Juba (South Sudan) were encouraging.
This strategy called CATI (for Case-Area Targeted Intervention) is now being evaluated in DRC, Cameroon and Zimbabwe to confirm its effectiveness.
Current evidence supports a high-risk spatiotemporal zone of 100 to 250 meters around case-households in which a case has been detected for 7 days. Based on national policies, local context, and operational considerations, MSF will decide on the content of the CATI kit to be deployed (water sanitation and hygiene measures at the household level, active case finding, antibiotic chemoprophylaxis and/or single-dose oral cholera vaccination, radius of intervention) and the prioritization strategy to be implemented. The Epicentre study will evaluate the effectiveness of the CATI strategy during an acute cholera outbreak, with clearly defined measures of the effectiveness of the CATI package. It will compare rapid response and the implementation of a "CATI kit" versus actions delayed by operational constraints. It will also estimate the feasibility, costs, and implementation process of this approach.
Diagnostics

Optimizing detection for faster intervention
The cholera rapid diagnostic tests (RDTs) are useful to speed-up the detection, confirmation and notification of cholera epidemics. Reliable estimates of the sensitivity and the specificity of RDTs are essential. Several analytical epidemiological studies conducted in Zambia, South Sudan and DRC have covered this question both for Crystal VC and SD Bioline RDTs, using the direct method with stools and also an enrichment step in alkaline peptone water.
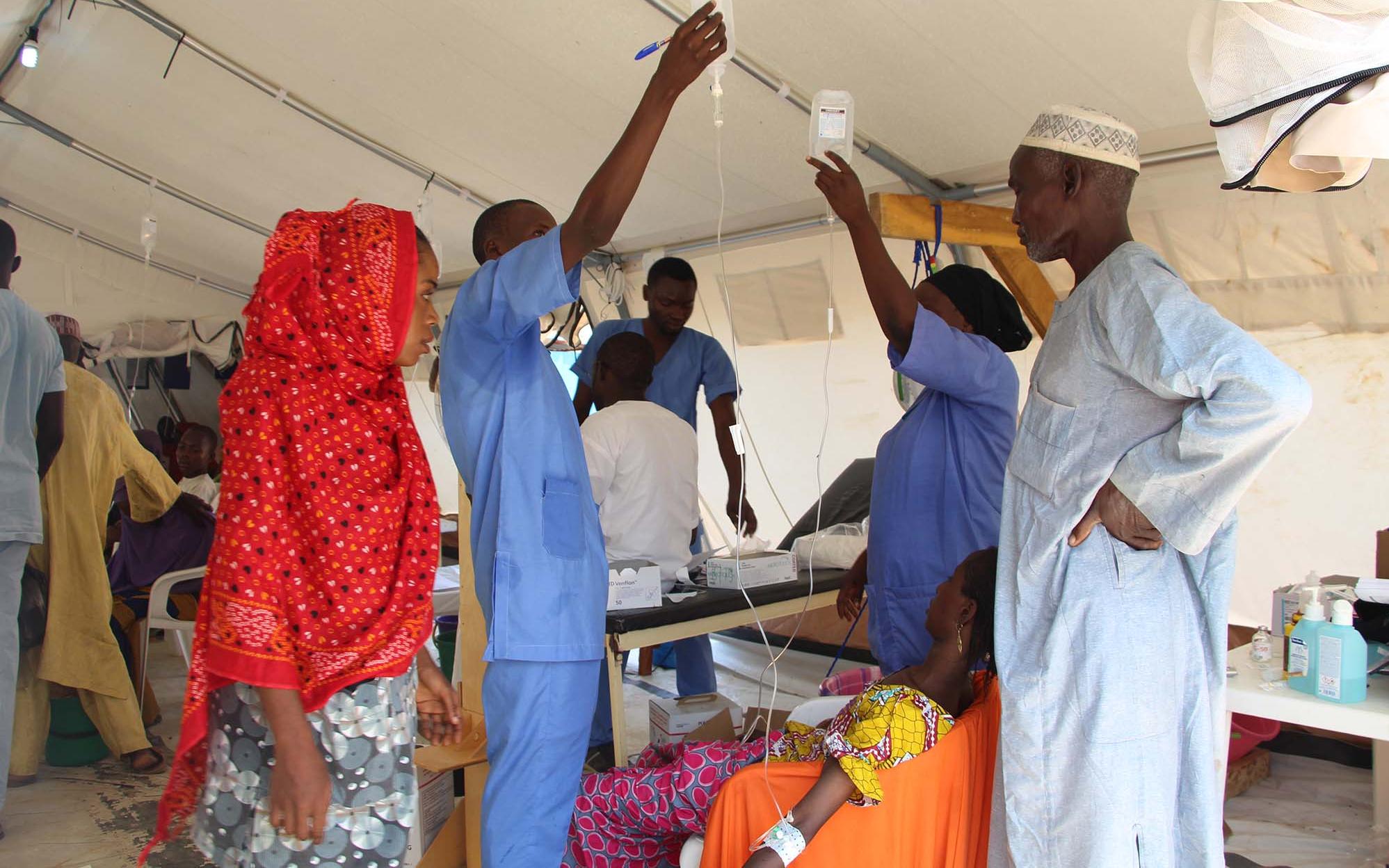
Cholera vaccination
Vaccination as a public health tool
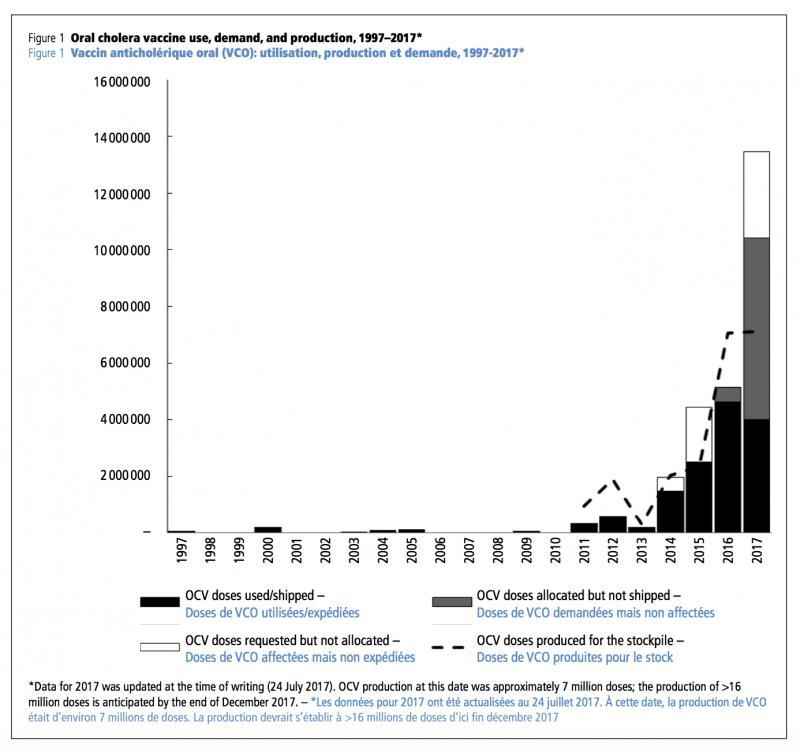
Before 2012, cholera vaccine was almost exclusively a traveler’s vaccine and not a public health tool. In 2012, MSF conducted the first vaccination campaign in Guinea with Shanchol, a newly WHO prequalified vaccine, and analytical and descriptive epidemiological studies conducted by Epicentre shown the feasibility of conducting such a campaign as well as the safety, efficacy, and acceptability of the vaccine5. This was an important step that contributed to the creation of the global cholera vaccine stockpile in 2013. Since then vaccination has become a standard tool in the fight against cholera. Now 3 oral vaccines requiring two doses to be fully effective are prequalified by WHO: Dukoral®, Shanchol™, and Euvichol®. The latter two are part of the global stockpile established by WHO with the support of Gavi, the Vaccine Alliance.
Vaccination in outbreaks
A timely response is essential during outbreaks, which is challenging to achieve with the currently recommended two-dose schedule. Epicentre is exploring the feasibility of controlling the outbreak spread by administering a single-dose. Results from our case-control studies conducted in Zambia and South Sudan indicated that the short term protection of single-dose is similar than two-dose protection in the short term6,7.
Hard-to-reach populations

Access to vaccination and the administration of a two-dose schedule is particularly problematic for hard-to-reach populations, such as fishermen or displaced population living in conflict areas. MSF has piloted administration of the 2nd dose either with the help of a community leader or at the individual level in such settings among fishermen in Malawi. This strategy has been made possible thanks to the high thermal-stability of the Shanchol vaccine , which has been recently licensed for controlled-temperature-chain (CTC) use, meaning the vaccine can be safely used for 14 days stored up to 40C. Vaccine coverage surveys showed good acceptability among fishermen in Malawi and an analytical study showed the high effectiveness of this strategy to prevent cholera.
Epicentre is part of the oral cholera vaccine working group at the Strategic Advisory Group of Experts (SAGE). Epicentre is also member of the Global Task Force for Cholera Control and actively participates in several working groups and chairs the cholera surveillance working group
Evaluating Vaccination Campaigns in DRC
Epicentre launched an operational study in spring 2021 to evaluate the impact of a preventive oral vaccination campaign in cholera hotspots in the DRC, with funding from the Wellcome, UK AID and Foreign and Commonwealth and Development Office, in conjunction with MSF activities and with the support of the DRC Ministry of Health and the National Program for the Elimination of Cholera and the Control of Other Diarrheal Diseases (PNECHOL-MD). The objective is to monitor the incidence of cholera after vaccination for at least two years, and to better understand the impact of the vaccine on the level and characteristics of disease transmission. The actual impact of preventive vaccinations is still poorly documented.
The combination of clinical surveillance, serological surveys, and monitoring of infected households will also contribute to a better understanding of the dynamics of cholera transmission in endemic settings and inform strategies to be put in place.
Selected resources
Case-area targeted interventions to rapidly contain the spread of cholera: updates from the DRC study | Nana Mimbu & Flavio Finger
Assessing cholera transmission in endemic areas following mass preventive OCV campaigns in DRC | Anaïs Broban
Selected publications
Inference is bliss: Simulation for power estimation for an observational study of a cholera outbreak intervention.
Effectiveness of case-area targeted interventions including vaccination on the control of epidemic cholera: protocol for a prospective observational study.
Regional sequencing collaboration reveals persistence of the T12 O1 lineage in West Africa.
Successive epidemic waves of cholera in South Sudan between 2014 and 2017: a descriptive epidemiological study.
High cholera vaccination coverage following emergency campaign in Haiti: Results from a cluster survey in three rural Communes in the South Department, 2017.
Effectiveness of oral cholera vaccine in preventing cholera among fishermen in Lake Chilwa, Malawi: A case-control study.
Mapping the burden of cholera in sub-Saharan Africa and implications for control: an analysis of data across geographical scales.
Cholera epidemic in Yemen, 2016-18: an analysis of surveillance data.
References
1 Lessler J, Moore SM, Luquero FJ, McKay HS, Grais R, Henkens M, et al. Mapping the burden of cholera in sub-Saharan Africa and implications for control: an analysis of data across geographical scales. Lancet 2018. doi:10.1016/S0140-6736(17)33050-7.
2 Weill F-X, Domman D, Njamkepo E, Tarr C, Rauzier J, Fawal N, et al. Genomic history of the seventh pandemic of cholera in Africa. Science (80- ) 2017;358. doi:10.1126/science.aad5901.
3 Luquero FJ, Rondy M, Boncy J, Munger A, Mekaoui H, Rymshaw E, et al. Mortality rates during cholera Epidemic, Haiti, 2010-2011. Emerg Infect Dis 2016;22. doi:10.3201/eid2203.141970.
4 Camacho A, Bouhenia M, Alyusfi R, Alkohlani A, Naji MAM, de Radiguès X, et al. Cholera epidemic in Yemen, 2016–18: an analysis of surveillance data. Lancet Glob Heal 2018;0. doi:10.1016/S2214-109X(18)30230-4.
5 Luquero FJ, Grout L, Ciglenecki I, Sakoba K, Traore B, Heile M, et al. Use of Vibrio cholerae vaccine in an outbreak in Guinea. N Engl J Med 2014;370. doi:10.1056/NEJMoa1312680.
6 Azman AS, Parker LA, Rumunu J, Tadesse F, Grandesso F, Deng LL, et al. Effectiveness of one dose of oral cholera vaccine in response to an outbreak: a case-cohort study. Lancet Glob Heal 2016;4:e856–63. doi:10.1016/S2214-109X(16)30211-X.
7 Ferreras E, Chizema-Kawesha E, Blake A, Chewe O, Mwaba J, Zulu G, et al. Single-Dose Cholera Vaccine in Response to an Outbreak in Zambia. N Engl J Med 2018;378. doi:10.1056/NEJMc1711583.