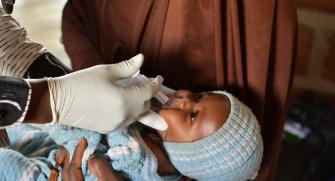
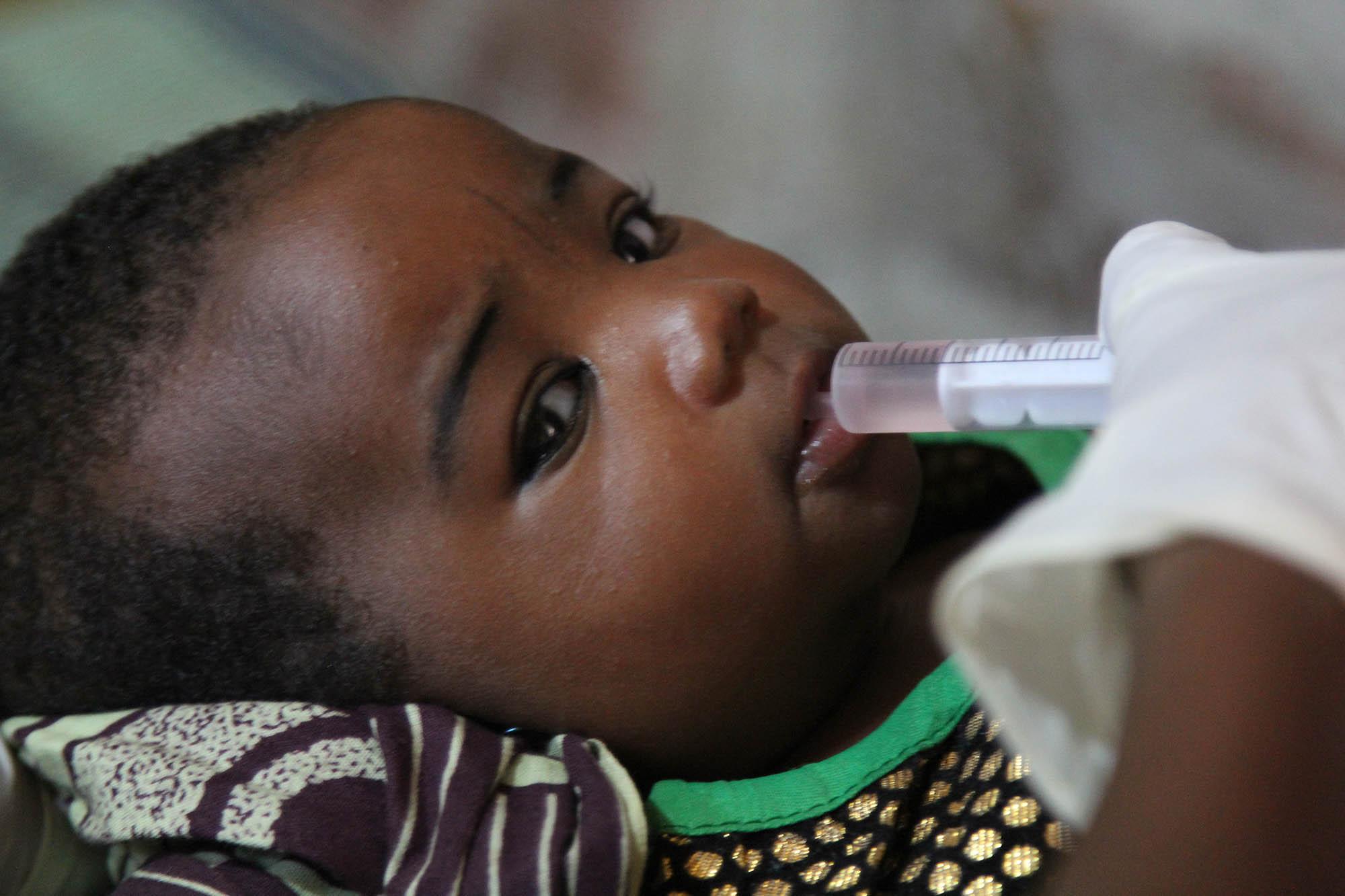
Rotavirus
A preventable cause of severe gastroenteritis
Acute diarrhea is rapidly dehydrating and can be life-threatening unless fluid therapy is initiated quickly. Treating severe rotavirus gastroenteritis involves hospitalization and rehydration therapy. In countries with rapid access to high-quality of care, children are taken under charge and almost always survive the infection. In the areas where Médecins Sans Frontières (MSF) works, however, diarrhea remains one of the major causes of childhood morbidity and mortality. Rotavirus is a major cause of diarrheal disease that can be prevented with a vaccine.
Developing a vaccine for difficult settings
Adapting to the context
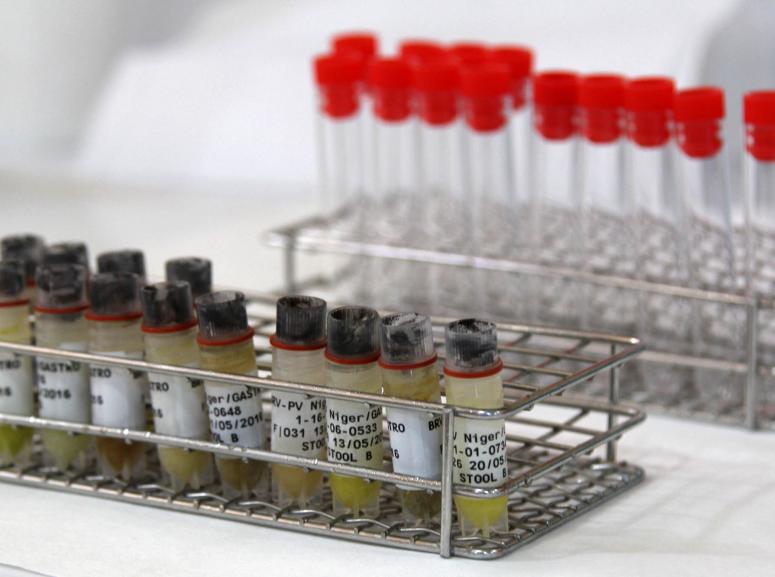
As of 2018, four vaccines are WHO prequalified, but they are not adapted to the settings where MSF works due to the logistical challenges of the large storage footprint and cold chain requirements. Epicentre has focused on research and development of a rotavirus vaccine that can be brought to the infants that need it most: a new vaccine that is easy-to-use and an affordable option for developing countries.The clinical trial coordinated in Niger by Epicentre on this vaccine contributed to its prequalification by the WHO.
Proven efficacy
The vaccine tested by Epicentre is called Rotasill and is manufactured by the Serum Institute of India Pvt Ltd. Although there are several other potential candidate vaccines that could expand access and respond to the needs, this vaccine was selected as the most promising. This vaccine is based on existing vaccines, and its proven safety track record minimizes risk.
Heat-stable, easy to transport and affordable
Rotasiil has several other properties that make it especially suitable for use in sub-Saharan Africa: it is heat stable and does not require a cold chain, this makes it suitable for places where maintaining the cold chain is a challenge. Less packaging and easier transport
are additional advantages. Last, but not least, this is an affordable vaccine.
Epicentre’s field and clinical trials
In an initial publication in 2018, Epicentre showed the efficacy of this vaccine to prevent severe gastroenteritis among infants in Niger. The results published in the New England Journal of Medicine show that the vaccine has no safety concerns with an efficacy of 66.8% in preventing severe gastroenteritis caused by rotavirus. It means that 6 children in 10 receiving the vaccine did not develop severe gastroenteritis. In a publication published in Plos Medicine in 2021, the follow-up of the children was extended to the age of 2 years and the effectiveness of the vaccine against severe forms of gastroenteritis was 60.3% after one year and 54.7% after two years.
This is more promising than the efficacy of existing rotavirus vaccines which were previously tested in Sub-Saharan Africa.
Prequalification
Rotasill vaccine was prequalified by WHO in 2018. This means it can now can be purchased by UN agencies and governments. Prequalification is a big step to ensure that this vaccine can be rolled out quickly in sub-Saharan Africa. MSF's aim is to ensure access to vaccines that require minimal logistics and that can be made available to populations in greatest need. This clinical trial has made a significant contribution to making that possible.
- Weekly epidemiological record WHO (https://apps.who.int/iris/bitstream/handle/10665/342904/WER9628-eng-fre.pdf