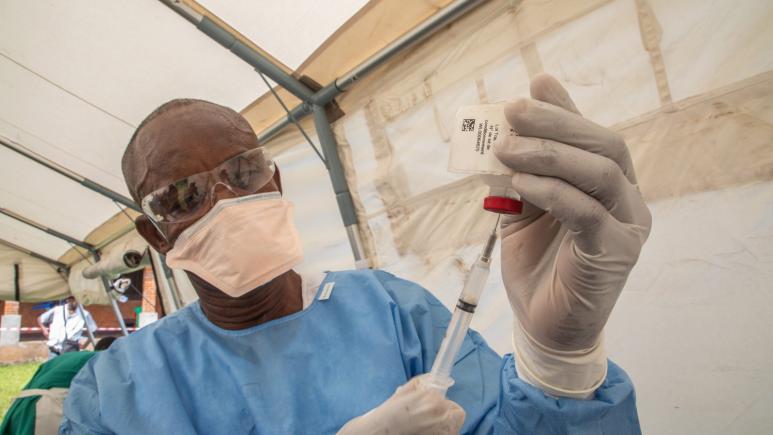
Ebola vaccine trial at Epicentre Mbarara Research Centre in Uganda
Objective of the trial
Ebola is a serious disease with a high case fatality rate ranging from 30 to 90%. Many outbreak episodes have occurred repeatedly in many African’s countries claiming the lives of thousands people. Due to the current outbreak going on in the neighbor country, the Democratic Republic of Congo, Uganda is among the countries which are at high risk of the disease spreading to its territory. Moreover, it has been extremely hard to make a quick response to an Ebola outbreak and no treatment is available to cure the disease. However, there is a hope that the vaccine against Ebola can help to control the disease. To date, no Ebola vaccine has been licensed to be used in a mass vaccination program. Therefore, there is a need to conduct research to generate scientific evidence that the vaccine is able to protect the vaccinated population or not. If, yes the study will lead to the vaccine being licensed and therefore can be used in the routine vaccination program to protect the general population.
Trial conduct
Despite the fact that Uganda is highly regulated country regarding clinical research, effort was made to get all the needed approvals for the study after a long submission process. The Site Initiation Visit (SIV) for the trial was done in mid - July 2019 leading to the site activation on 31 July 2019. The recruitment of participants has started on 1st August 2019 and so far 305 participants have been included in the study for a total targeted seize of 800 participants. No major obstacle/issue was observed since the recruitment started. All the participants will receive 2 doses, the first dose given the day of inclusion (Day 0) and the second 56 days later. Among the 305 participants included, 94 have received the second dose. All the follow-up visits are being done successfully to date. In addition to the vaccination process, the study has qualitative component aiming to do individual interview and focus group discussion for a total of 70 vaccinated participants. Currently, 33 participants have attended the individual interview. Another sub-study about PBMC (Peripheral Blood Mononuclear Cells) process to get additional information on the immunogenicity is about to start very soon.
This is among the most important studies for Epicentre that will allow having a vaccine marketed in order to control the devastating outbreak that has created losses of thousands of lives.
Related resource
See allEpicentre Uganda
Find out more