Malaria
Malaria: origin and transmission
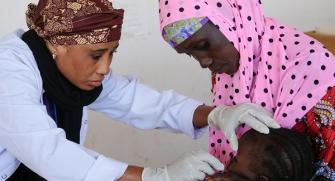
Malaria is caused by single-celled Plasmodium parasites that are transmitted to humans by mosquitoes. In 2019, there were an estimated 229 million malaria infections and 409 000 malaria deaths worldwide1. Africa alone accounts for more than 80% of cases and more than 90% of deaths, and the disease continues to affect mostly children and pregnant women and children under 5 years old2.
In the early 2000s advances were made such as the roll-out of insecticide-treated bednets and the introduction of artemisinin-based combination therapy (ACT). Thanks to these interventions the number of deaths declined significantly between 2000 and 2015 and the incidence rate (cases per 1,000 inhabitants exposed to the risk of malaria) has dropped from 80 in 2000 to 57 in 20192, but they have recently plateaued. Malaria has been the focus of Médecins Sans Frontières (MSF) for many years: 2 730 600 cases of malaria were treated by MSF in 2020, representing one-quarter of MSF outpatient consultations worldwide2, which is an obvious justification for Epicentre’s involvement in this field.
Epicentre’s contributions have been focused on the diagnosis, treatment and prevention of malaria in MSF program settings in many different fields.
Seasonal malaria chemoprevention (SMC) and other mass drug distributions

Epicentre accompanied MSF during the roll-out of seasonal malaria chemoprevention (SMC), a new strategy aimed at children under 5 in the Sahel. They are given prophylaxis drugs once a month during the rainy season when risk of malaria is high3 . Epicentre has carried out program coverage surveys of MSF-implemented SMC in Guinea-Bissau, Mali, Niger, and Chad, and provided advice on the continued roll-out of SMC4.
After the roll-out of the SMC strategy in Magaria, Niger, doubts arose about the program’s effectiveness, as the hospitals were still full of children with malaria. In 2016, Epicentre conducted a prospective case-control study to estimate the protective effectiveness of SMC against developing malaria. Because one of the concerns was that children were not correctly adhering to the 3-day course of treatment at home, the blood levels of the SMC drugs were checked. The main results showed that SMC is generally working well despite evidence of very poor adherence to the drug treatment.
To protect vulnerable population, MSF organized a preventive treatment program for children <15 years in a refugee camp in northern Uganda. In 2015, intermittent preventive treatment of malaria (IPTc) was implemented in two refugee camps among children aged 6 months to 14 years. Three distributions of dihydroartemisinin-piperaquine (DP) were conducted 8 weeks apart.
Epicentre conducted a series of studies to describe this intervention. The knowledge gained should inform operational responses to crises of displaced persons both in Uganda and other high malaria transmission settings.

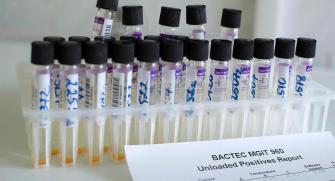
One of the risks of SMC, is that the malaria parasites may become resistant to the drugs used in the SMC strategy. The World Health Organization has recommended that routine monitoring of this takes place in areas where SMC is implemented. To do this, laboratory methods are used to look at the genes of the malaria parasites, and to see which ones might be resistant to the drugs. Epicentre has performed studies in Chad and Niger on this subject.
In Niger, the study showed that parasites with genotypes associated with the highest levels of resistance to amodiaquine and sulfadoxine-pyrimethamine are not yet common. However, it seems important to continue monitoring these resistance markers. This study also showed the heterogeneity of the parasites in a rather small area and the need to be cautious when extrapolating the results of surveys on molecular markers of resistance in a single site to deduce actions.
In addition, a study is underway in Chad in the Moïssala district to evaluate genetic markers of sulfadoxine-pyrimethamine and amodiaquine resistance and to monitor their evolution since the beginning of the implementation of the SPC in 2012. The 1st analysis was carried out before the 1st distribution and then in 2014 and in 2021. First signs of resistance had been able to be highlighted at the end of the study in 2014, it is therefore necessary to verify if they are confirmed in order to quickly identify the emergence of resistance to the currently used treatments and propose adapted therapeutic responses quickly.
Improving diagnostics
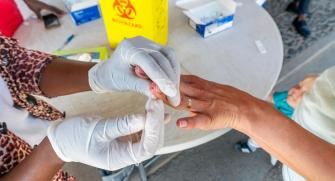
Rapid diagnostic tests (RDT) for malaria do not require specialist laboratory capacity, and are widely available in the field. Epicentre performed evaluations of several different types of rapid diagnostic tests (notably HRP2 and pLDH), to see which are best-adapted to different settings. One study in Uganda showed that HRP2 tests continued to give a positive result for up to 6 weeks after a treatment7, suggesting that they should be avoided in high transmission areas where early re-infection in common.
Developing better treatment strategies
Field and clinical trials
Epicentre has performed and participated in several recent clinical trials related to the treatment of malaria.
- In Niger, one study evaluated three commonly-used ACTs - Artemisinin-based combination therapy - for the treatment of uncomplicated malaria, and found that all three had good effectiveness.
- The treatment of malaria in malnourished children can be challenging. In a clinical trial in Mali and Niger, artemether-lumefantrine was found to be equally effective in malnourished and non-malnourished children, but the levels of the drugs in the blood were significantly lower in malnourished children, possibly leaving them more vulnerable to reinfection9.
- Epicentre was one of 11 trial sites for the AQUAMAT trial, which showed that intravenous artesunate therapy reduced mortality from severe malaria in children by 22% compared to quinine treatment. This trial led to a change in WHO recommendations for the treatment of children with this extremely lethal form of malaria.
DeTACT to uncover new therapeutic approaches
Epicentre's Niger Centre is one of 14 sites - 8 countries in Africa and 5 in Asia - of the DeTACT project coordinated by MORU and funded by UKaid and the UK Foreign and Commonwealth Development Office (FCDO). It aims to discover new therapeutic approaches against malaria. Indeed, resistance to new drugs combining an artemisinin derivative with one of the many anti-malarial molecules has started to appear in Asia and is now spreading in some regions of South East Asia. The DeTACT project is studying the efficacy, safety and tolerability of two artemisinin-based combination therapies (ACTs) using existing antimalarial drugs (artemether-lumefantrine + amodiaquine and artesunate-mefloquine + piperaquine).
Find out more
Efficacy and safety of Triple ACTs compared to conventional ACTs for the treatment of uncomplicated malaria: Preliminary results of the DeTACT trial in Niger
Mass drug administration as a tool for rapid reduction of malaria morbidity and mortality in emergency settings
Incidence of Malaria, with or without Seasonal Malaria Chemoprevention (SMC) in Moïssala, Chad 2014-2021
Evidence and new recommendations for malaria prevention tools
Selected publications
Evaluation of HRP2 and pLDH-based rapid diagnostic tests for malaria and prevalence of pfhrp2/3 deletions in Aweil, South Sudan.
Seasonal malaria chemoprevention: successes and missed opportunities.
Intermittent preventive treatment for malaria among children in a refugee camp in Northern Uganda: lessons learned.
Molecular markers of resistance to amodiaquine plus sulfadoxine-pyrimethamine in an area with seasonal malaria chemoprevention in south central Niger.
Performance and time to become negative after treatment of three malaria rapid diagnostic tests in low and high malaria transmission settings.
Efficacy of artesunate-amodiaquine, dihydroartemisinin-piperaquine and artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Maradi, Niger.
Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial.
References
1 WHO. https://www.who.int/fr/news-room/fact-sheets/detail/malaria
2 WHO. https://cdn.who.int/media/docs/default-source/malaria/world-malaria-reports/world-malaria-report-2020-briefing-kit-fre.pdf?sfvrsn=69c55393_9
3 WHO Global Malaria Programme. WHO policy recommendation: Seasonal Malaria Chemoprevention for Plasmodium falciparum control in highly seasonal transmission areas of the Sahel sub-region in Africa. Geneva; 2012.