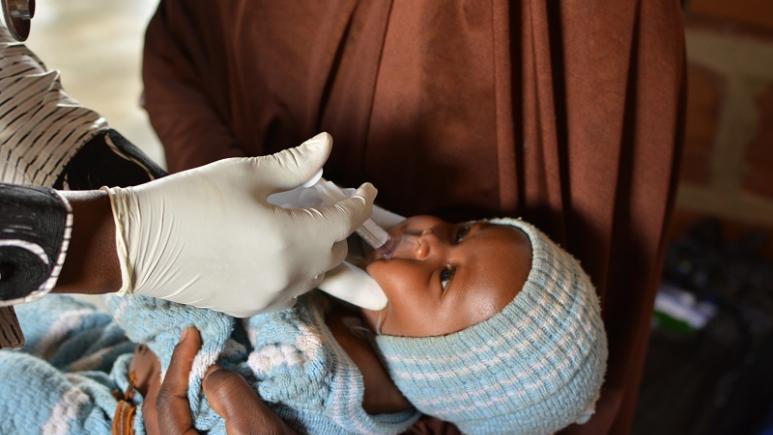
First heat-stable rotavirus vaccine recommended by WHO
Rotavirus is the most common cause of severe diarrheal disease in young children in the world, with the majority of disease and deaths due to rotavirus occurring in high-mortality, low-income countries. Although there is no specific treatment, there are currently 4 live, oral attenuated vaccines which are licensed and prequalified by WHO. The goal of WHO vaccines prequalification is to ensure that vaccines used in immunization programmes are safe and effective. Prequalification also supports the specific needs of national immunization programmes. In order to be delivered to children in need where infrastructure to deliver vaccines may be week, formulations which do not require the classic cold chain are essential. In a new report, Epicentre and partners confirm the sustained efficacy obtained with Rotasiil®, an oral vaccine that can be delivered out of cold chain, which is a real advantage for low- and middle-income countries. Sheila Isanaka, an epidemiologist with Epicentre and principal investigator of the trial, tells us more about rotavirus and the benefits of this vaccine
How can we protect children from rotavirus infections and the serious diarrheal diseases they can cause?
Sheila Isanaka: Rotavirus vaccine is one of the few proven ways to protect children against rotavirus disease, a leading cause of severe gastroenteritis among young children. Rotavirus continues to substantial cause morbidity and mortality, although the total number of deaths related to diarrhea has decreased, especially since the introduction of vaccines. Between 125,000 and 200,000 deaths are attributable to rotavirus each year worldwide (1).
To reduce this substantial burden, prevention through vaccination is essential, and the World Health Organization (WHO) recommends rotavirus vaccine use in all countries. A 40% reduction in rotavirus prevalence was observed after the introduction of the vaccine (2). Four rotavirus vaccines are prequalified by WHO: Rotarix®, RotaTeq® and since 2018, two new vaccines have been prequalified Rotavac® (Bharat Biotech, Hyderabad, India, Rotasiil® (Serum Institute of India PVT. LTD., Pune, India).
WHO Pre-qualification means that these vaccines can now be purchased by UN agencies and governments. About 100 countries have introduced rotavirus vaccine into their national immunization programs. However, RotaTeq® is not available through GAVI support, which reduces its accessibility for African countries.
What are the differences between the available pre-qualified vaccines?
S.I.: They are all oral vaccines, easy to administer with similar efficacy and safety profiles. Rotasiil® has the unique feature of being heat-stable. It remains stable at high temperatures and does not require a cold chain, which is a real advantage for sub-Saharan Africa, where cold chain infrastructure is limited. It has the additional advantage of requiring less packaging which makes easier to store and transport to the point of delivery. Last but not least, it is an affordable vaccine.
What was the efficacy of this vaccine in the Phase III trial you coordinated in Niger?
SI.: In our first, primary analysis, we found this oral rotavirus vaccine to have an efficacy of 66.7% against severe rotavirus gastroenteritis among infants in Niger at 28 days after the third dose of vaccine (3).
In our latest publication in Plos Medicine with extended follow up to 2 years of age, we were able to show that efficacy of the vaccine against severe rotavirus gastroenteric was 60.3% after one year and 54.7% after two years.
The estimation of the efficacy of Rotasiil® against severe rotavirus gastroenteritis during the first year of life (60.3%) is similar to that of other oral rotavirus vaccines assessed in other high- mortality countries. In the recently updated WHO rotavirus vaccine position paper (2), all four vaccines were endorsed and recommended. Over a two-year follow-up, the corresponding reduction in severe rotavirus gastroenteritis when comparing Rotasiil® to placebo with placebo was 44% (95% CI 26% to 58%) pooling the trial in Niger and an additional confirmatory trial in India.
Supply shortages have slowed the introduction of rotavirus vaccines in low resources countries. With these results, which were used to support the recent WHO updated recommendations, as well as the vaccine’s heat stability and presentation provides an important option for Sub-Saharan Africa.
1. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years
Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, Kang G, Kirkpatrick BD, Kirkwood CD, Mwenda JM, Parashar UD, Petri WA Jr, Riddle MS, Steele AD, Thompson RL, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC Jr.JAMA Pediatr. 2018 Oct 1;172(10):958-965. doi: 10.1001/jamapediatrics.2018.1960.
2. Weekly epidemiological record WHO (https://apps.who.int/iris/bitstream/handle/10665/342904/WER9628-eng-fre.pdf
3. Efficacy of a Low-Cost, Heat-Stable Oral Rotavirus Vaccine in Niger. Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, Sayinzoga-Makombe N, McNeal MM, Meyer N, Adehossi E, Djibo A, Jochum B, Grais RF. N Engl J Med. 2017 Mar 23;376(12):1121-1130. doi: 10.1056/NEJMoa1609462.
Read the publication
Rotavirus vaccine efficacy up to 2 years of age and against diverse circulating rotavirus strains in Niger: Extended follow-up of a randomized controlled trial.
Find out more on rotavirus
Find out more
Niger Research Center
Find out more