[Immersion] in the clinical trial on the administration of a delayed second dose of cholera vaccine in Guinea
Background
A disease of poverty: cholera
 Cholera is one of the oldest diseases affecting humans, who are the main natural hosts of the Vibrio cholerae bacteria that cause this infectious disease. Vibrio cholerae is also present in the environment. This disease affects vulnerable populations living in unsanitary conditions with insufficient health facilities. Every year, according to WHO estimates (1), there are between 1.4 and 3 million cases of cholera in the world and between 21,000 and 143,000 deaths, mainly in the poorest regions of the world.
Cholera is one of the oldest diseases affecting humans, who are the main natural hosts of the Vibrio cholerae bacteria that cause this infectious disease. Vibrio cholerae is also present in the environment. This disease affects vulnerable populations living in unsanitary conditions with insufficient health facilities. Every year, according to WHO estimates (1), there are between 1.4 and 3 million cases of cholera in the world and between 21,000 and 143,000 deaths, mainly in the poorest regions of the world.
An uncontrolled disease
 Cholera prevention requires access to safe drinking water, adequate sanitation, good hand hygiene and, in the event of an outbreak, vaccination to stop the spread of the bacteria. Despite this, cholera is far from being controlled. Once a case is suspected, it must be reported, and measures must be taken to stop the spread of the disease. In addition to patient management, these measures include raising awareness of the population, distributing water treatment products, and improving access to safe drinking water. Vaccination is a complementary measure in the fight against cholera.
Cholera prevention requires access to safe drinking water, adequate sanitation, good hand hygiene and, in the event of an outbreak, vaccination to stop the spread of the bacteria. Despite this, cholera is far from being controlled. Once a case is suspected, it must be reported, and measures must be taken to stop the spread of the disease. In addition to patient management, these measures include raising awareness of the population, distributing water treatment products, and improving access to safe drinking water. Vaccination is a complementary measure in the fight against cholera.
A vaccine to stop the spread of cholera outbreaks
There are currently three WHO prequalified oral cholera vaccines. The manufacturers recommend two doses with an interval of 7 to 14 days. All three vaccines show good efficacy. Two of these oral vaccines are particularly well suited for use in cholera-endemic countries because of their affordability, relatively simple manufacture, and ease of transport and storage.
.
2018 | An observation: a vaccination schedule poorly adapted to emergency contexts
The use of these oral cholera vaccines provides significant improvements in preventing and containing outbreaks. However, in emergency context, it is rarely feasible to carry out two rounds of vaccination campaigns within a short delay of two weeks. Indeed, outbreaks most often occur in contexts of insecurity or natural disasters, which limits the possibility of administering the second dose in the allotted time. Vaccine production limitations can also be a constraint to administering a second dose within a 2-week period. In many vaccination campaigns to date, the second dose has been administered with a longer interval. Therefore, single-dose vaccination strategies are sometimes proposed in response to an outbreak to achieve higher vaccination coverage with the same number of doses. But the second dose is still needed to build up immunity for a longer period of time.
Project itinerary
2019 | Defining the optimal interval between two doses
It is therefore important to assess the impact of increasing the interval between doses in terms of protection against cholera. Observational data even suggest that a delayed administration of the second dose appears to provide at least equivalent protection. Other research even suggests that the 14-day period is too short for the second dose to boost immunity. Only a proper clinical trial in an area where people have never been vaccinated will provide strong scientific evidence on this point.
“In collaboration with the Ministry of Health and representatives of the Guinean health authorities, MSF, with the support of Epicentre, has decided to launch a clinical trial to demonstrate whether the immune response following a prolonged interval of 6 or 12 months between two doses of oral cholera vaccine is at least as good as that observed when administering the second dose of vaccine according to the manufacturer's instructions, i.e., two weeks after the first dose, explains Fabien Kabongo, MSF's medical coordinator in Guinea. We hope to be able to define the optimal conditions for administering the vaccine and to improve the fight against cholera outbreaks in Guinea and more broadly throughout the world.”
Funding from the Grieg Foundation has allowed laying the foundations for this randomized clinical trial which will take place in Conakry, the capital of Guinea.
Setting up a clinical research project requires rigour and perseverance
 "From its conceptual stage to its implementation, a clinical trial requires many steps, as well as national and international collaborations to establish. Research must comply with ethical principles to guarantee the safety and protection of participants and must follow a rigorous methodology to ensure the reliability and reproducibility of results. Only this strict framework will make it possible to obtain sufficiently strong scientific results to influence medical practices, in our case for preventive care, and thus improve the care of populations. The research protocol determines this framework: it defines notably the objectives of the study, the criteria for selecting participants, the follow-up procedures, the methodology necessary for data analysis, particularly statistical, the ethical framework, and the management and protection of data. The study will thus involve many people with specific skills. For example, throughout the trial, adverse events occurring in a participant must be identified, managed and reported. A doctor has therefore been specially recruited and trained for this purpose, and a reference health structure has also been identified for the follow-up of the participants. This means a substantial human capacity building for research, with the hope that the trained individuals will then contribute to other research projects to further improve the health of the populations,"
"From its conceptual stage to its implementation, a clinical trial requires many steps, as well as national and international collaborations to establish. Research must comply with ethical principles to guarantee the safety and protection of participants and must follow a rigorous methodology to ensure the reliability and reproducibility of results. Only this strict framework will make it possible to obtain sufficiently strong scientific results to influence medical practices, in our case for preventive care, and thus improve the care of populations. The research protocol determines this framework: it defines notably the objectives of the study, the criteria for selecting participants, the follow-up procedures, the methodology necessary for data analysis, particularly statistical, the ethical framework, and the management and protection of data. The study will thus involve many people with specific skills. For example, throughout the trial, adverse events occurring in a participant must be identified, managed and reported. A doctor has therefore been specially recruited and trained for this purpose, and a reference health structure has also been identified for the follow-up of the participants. This means a substantial human capacity building for research, with the hope that the trained individuals will then contribute to other research projects to further improve the health of the populations,"
Fabienne Nackers, investigator who is collaborating with the team in the implementation of the trial.
Why Conakry?
Conakry is a port city with an estimated population of about 2 million in 2019. Cholera is endemic in Guinea, where outbreaks have been documented in 1994, 2006-2007 and a most recent larger outbreak in 2012. In 2012, 7350 cases were reported, two thirds of which were in Conakry. Therefore, the populations of Conakry and Guinea will certainly benefit from the results of this study.
Guinean stakeholders involved in the fight against cholera have shown their interest in using this vaccination. Indeed, the first response to a cholera outbreak using an oral vaccine in Africa was implemented in Guinea (specifically vaccinating in 2 prefectures: Boffa and Forécariah) as part of the response to the 2012 cholera outbreak. The documentation of this reactive mass vaccination has led to a fruitful and challenging scientific collaboration between MSF, the sponsor of this study, Epicentre and the Guinean investigators.
2020-2022 | Setting up the infrastructure to conduct such research

"Before starting the study, the team had to be assembled and trained. We needed people to recruit participants, which requires explaining the study to them, obtaining their consent, collecting samples, administering the vaccines, and then following them up to the end of the trial. In addition to their medical skills, these people need to be trained in the principles of 'Good Clinical Practice', which guide the rigorous application of research methods and ensure that the ethics and rights of trial participants are protected. We also had to recruit data managers and logisticians to set up the necessary infrastructure at the participant recruitment site and in the laboratory, including ultra-cold chain sample storage. The Epicentre team also includes several experts, notably in clinical trial methodology, laboratory methods, pharmacovigilance, statistics and data protection. Several agreements must be established with Guinean partners and international laboratories to conduct such a project. And throughout this preparation phase, we had to adapt to the uncertainties of the Covid-19 pandemic, which generated many delays, without limiting our enthusiasm."
Aboubacar SOUMAH, study coordinator
2021 - early 2022 | Bringing a laboratory out of the ground
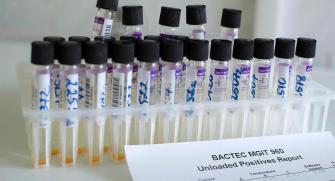
Blood samples are collected at different times during the study for serological analysis to notably assess the presence of vibriocidal antibodies, the best indicator of cholera immunity. "All samples are centrifuged, and the plasma are aliquoted in microtubes in a temperature-controlled environment. They are stored in a freezer at -20°C within 6 hours of collection, and then stored at -80°C. We therefore had to bring in an ultra-freezer (-80°C freezer).
For each participant, the plasma samples are then be shipped to our partner laboratory in the USA using a -80°C cold chain, in accordance with International Air Transport Association regulations for shipping biological material for serological testing.
May 22: the laboratory is finally operational

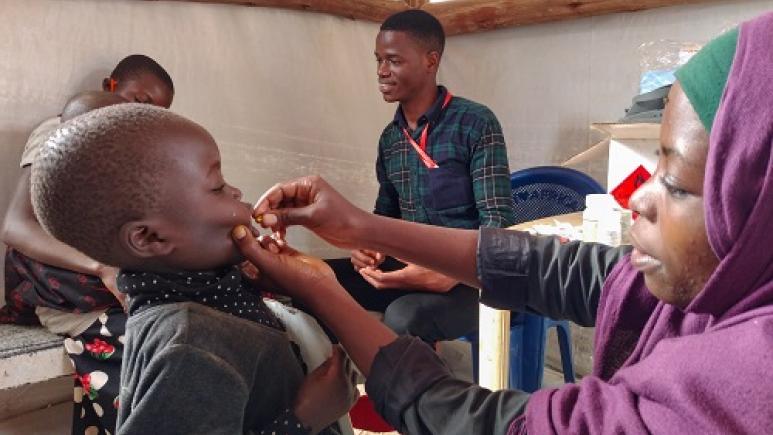
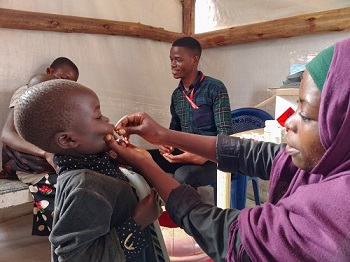
2022 | Recruiting participants and obtaining their consent
The community engagement team, comprising health promoters, is responsible for identifying and recruiting study participants, following local procedures. They organize information sessions on the study in health facilities and in the community.
Prior to any study-specific procedure, written informed consent is obtained from the participant or a representative (mother, father, or primary caregiver) for minors. If the participant is illiterate, consent is obtained in the presence of an impartial witness who is not related to the study team. In all, 456 people aged 1 to 39 years are to be recruited.
July 2022: first vaccination in the study
2022-2024 | Participant follow-up
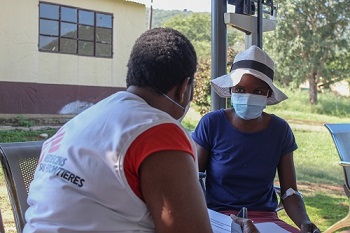 Participant follow-up is a challenge in the Conakry context. Participants will be contacted regularly by telephone by a member of the study team during their follow-up to remind them of their participation in the study, particularly the visits scheduled for the second dose of vaccine, 6 or 12 months after the first. The team will continue to follow all participants, with their agreement, until the end of the study, which for some is up to 18 months after enrolment.
Participant follow-up is a challenge in the Conakry context. Participants will be contacted regularly by telephone by a member of the study team during their follow-up to remind them of their participation in the study, particularly the visits scheduled for the second dose of vaccine, 6 or 12 months after the first. The team will continue to follow all participants, with their agreement, until the end of the study, which for some is up to 18 months after enrolment.
2022-2024 | Data analysis
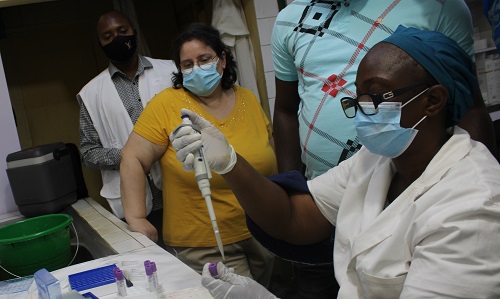 Throughout the study, data will be collected in accordance with confidentiality standards. Their quality will be checked to identify and correct erroneous, inaccurate, or irrelevant data. The data can then be analyzed to assess the non-inferiority of the humoral immune response between individuals receiving a second dose of vaccine 6 or 12 months after the initial dose compared to those who received a second dose 14 days after the first.
Throughout the study, data will be collected in accordance with confidentiality standards. Their quality will be checked to identify and correct erroneous, inaccurate, or irrelevant data. The data can then be analyzed to assess the non-inferiority of the humoral immune response between individuals receiving a second dose of vaccine 6 or 12 months after the initial dose compared to those who received a second dose 14 days after the first.
Finally
Only after completion of these different steps, reliable scientific data will make it possible to determine whether the humoral immune response is non-inferior between these different oral cholera vaccine schedules. If so, a longer interval between doses could then be considered as an alternative to the currently recommended 14-day interval.
(1) https://www.who.int/fr/news-room/fact-sheets/detail/cholera
crédit photo : Alawiya Mohammed, Moses Sawasawa, DR, Jakub Hein, Mohamed Bah