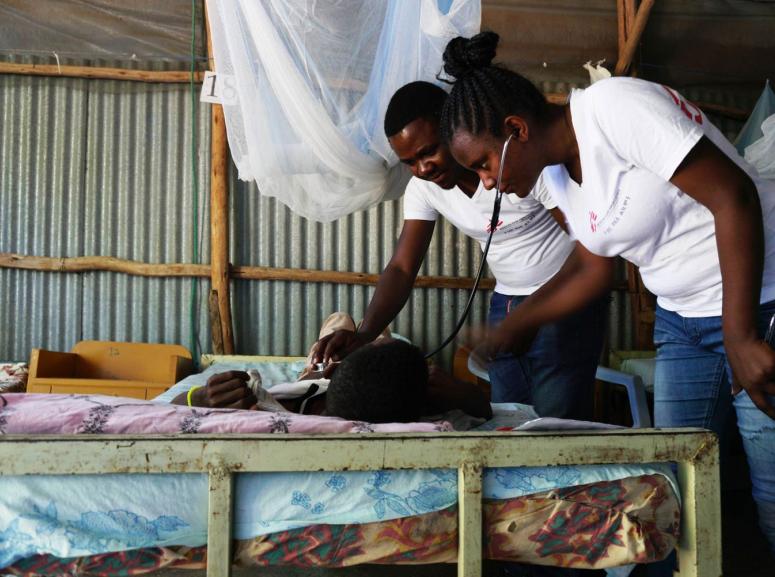
Visceral leishmaniasis
Kala-azar, a neglected parasitic disease
There are 3 main forms of leishmaniasis: the cutaneous form, the most frequent, is characterized by ulcerated lesions, in the cutaneous-mucosal form, ulcerations at the level of the mouth, nose and throat appear and the visceral leishmaniasis often called kala-azar which means black evil is the most severe.
Visceral leishmaniasis (VL) is characterized by fever, fatigue, enlarged liver and spleen, weight loss, and anemia. Transmitted through the Phlebotomus sandfly bite, the Leishmania protozoa causes. Without treatment, the outcome of visceral leishmaniasis is fatal.
Approximately 50,000-90,000 new visceral leishmaniasiscases occur annually and most of it in Brazil, East Africa and India.1 Visceral leishmaniasis epidemics affect the poorest populations, often living in unsanitary conditions. Environmental and climatic conditions, drug-resistant strains, immune suppression linked to malnutrition and HIV co-infection, and population movements contribute to Visceral leishmaniasis epidemics.
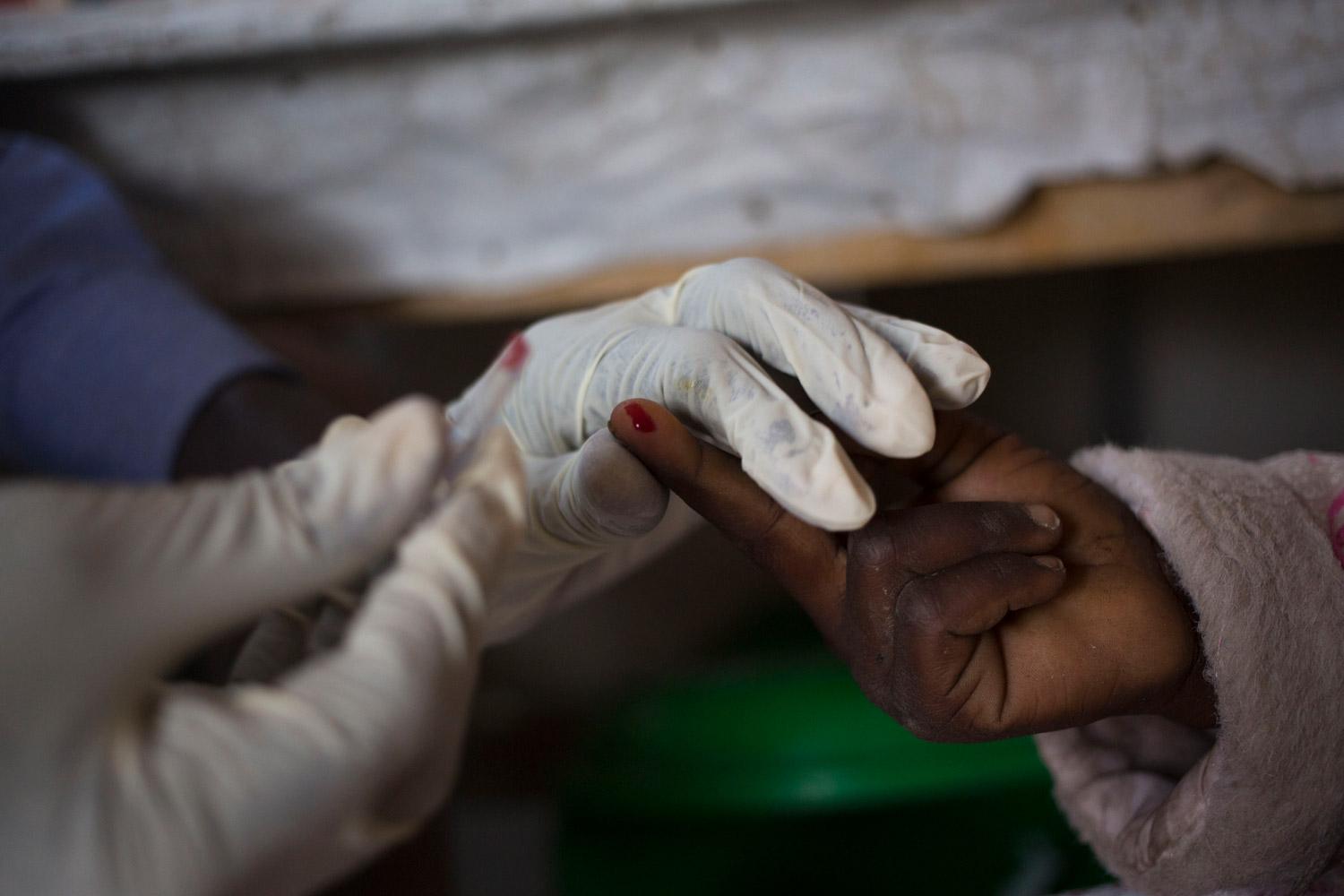
Visceral leishmaniasis diagnosis relies on serological and parasitological assessment (i.e., rapid tests and microscopy of spleen, bone marrow and lymph node aspirates). If the immune system is able to fight off the infection, the person is immune to further attacks. If not, the person becomes ill and the parasite spreads to the bone marrow, spleen, liver and lymph nodes. Visceral leishmaniasis is accompanied by a blackening of the skin, hence the name "mal noir" or kala-azar.
Treatment of Visceral leishmaniasis varies according to host and parasitic factors, but also the regions. As the treatments do not eliminate the parasite from the body, relapses occur. Without treatment, Visceral leishmaniasis is usually fatal. Delays in detection and treatment increase the risk of morbidity and mortality as well as the dissemination of disease to others.
Because visceral leishmaniasis develops at the expense of the immune system, people living with HIV who have a compromised immune system are at greater risk of developing severe forms of the disease. Unfortunately it is one of the most difficult opportunistic diseases of AIDS to treat.
In addition, visceral leishmaniasis weakens the immune system, so people with it are more likely to contract other opportunistic diseases such as malaria or tuberculosis.
Technical support to help vulnerable populations affected by Visceral Leishmaniasis
Epicentre provides technical support and conducts research on Visceral leishmaniasis. Technical support includes participation in the Médecins Sans Frontières’ (MSF) Neglected Tropical Diseases Working Group and the provision of advice and expertise, because of its expertise and studies in the field.
Ethiopia, reaching mobile workers

The estimated incidence of symptomatic vwledge of the barriers to visceral leishmaniasis diagnosis and care exist, particularly for the approximate 250 000 workers that migrate annually to the northwest region of the country for agricultural work.3 In collaboration with partners, Epicentre conducted a qualitative study to understand these barriers and guide strategies to increase access to Visceral leishmaniasis care among mobile workers.
In 2017, 50 in-depth interviews and 11 focus group discussions were conducted with mobile workers, visceral leishmaniasis patients and caretakers, healthcare workers, and community leaders in Kafta Humera District, Tigray, Ethiopia. Participants reported high vulnerability to visceral leishmaniasis among mobile workers and residents engaged in transitory work. Inadequate healthcare worker training, diagnostic test kit unavailability at the primary healthcare level, lack of Visceral leishmaniasis awareness, insufficient finances for care-seeking and prioritization of income-generating activities are significant barriers to diagnosis and care.

Social decision-making and financial support strongly and positively influence care-seeking; workers unable to receive salary advances, compensation for partial work, or peer assistance for contract completion are particularly disadvantaged. Participants recommended that authorities intervene to ensure: mobile workers access to bed-nets, food, shelter, water, and healthcare at farms or sick leave; decentralization of diagnostic tests to primary healthcare facilities; surplus medications/staff during the peak season; improved referral/feedback/reporting/training within the health system; free comprehensive healthcare for all VL-related services; and community health education.
Ultimately, this study provided guidance for urgently needed interventions tailored to the socio-economic and health needs of mobile workers (and other persons suffering from Visceral leishmaniasis) to reduce health disparities and the Visceral leishmaniasis burden.
Sudan, understanding prevention
While extensively studied in Asia4,5 limited data exist on the individual risk factors for visceral leishmaniasis transmission in Sudan. In 2012-2013, along with partners, Epicentre conducted a case-control study to identify individual- and household-level determinants of primary visceral leishmaniasis in 24 highly-VL-endemic villages in Gedaref State, Sudan. The study identified children and men as at higher risk for VL, and a reported visceral leishmaniasis patient(s) in the household in the prior year as the strongest risk factor. In multivariate analysis, visceral leishmaniasis risk increased with sleep location (outside the yard), household size, evening outdoor activities during the rainy season, use of ground nut oil and Acacia seyal smoke as repellents, presence of dogs in the yard at night, and proximity of Acacia nilotica forests. Drinking water sources other than the village water tank, a buffer distance from the yard of the adjacent house, and the presence of animals other than dogs in the yard at night appeared to decrease Visceral leishmaniasis risk.
Uganda and Kenya, mapping leishmania’s regional variation
In Uganda and Kenya, Epicentre conducted a retrospective analysis of routinely-collected clinical data from an MSF-supported hospital 17. The study found no marked seasonal or annual fluctuations in Visceral leishmaniasis, with males 5-14 years old most affected. Evaluation of an rK39 antigen-based rapid test as a first-line diagnostic found it comparable to the direct agglutination test and spleen aspiration, though low sensitivity remained a concern. First-line treatment with sodium stibogluconate resulted in low case-fatality rates and few relapses.
Importantly, this study found that the epidemiological and clinical features of Visceral leishmaniasis on the border between Uganda and Kenya differed greatly from Sudan, underscoring the importance of Visceral leishmaniasis research across different regions.
LeichAcess, a consortium to speed up the fight against and elimination of visceral leichmaniasis
Epicentre is a member of the LeichAcess consortium. This three-year project seeks to improve access to care for leishmaniasis patients, including vulnerable groups, for the various forms of the disease (visceral leishmaniasis, cutaneous leishmaniasis, PKDL, and VL/HIV). It is being implemented by a consortium made up of experts and key health institutions leading access within Ethiopia, Kenya, South Sudan, Sudan, and Uganda.
LeishAccess will increase the use of optimal diagnostics and treatment combinations to treat visceral leishmaniasis (VL), VL/HIV co-infection, and post-kala-azar dermal leishmaniasis (PKDL), a common complication that appears after visceral leishmaniasis treatment. The project will also improve access to the treatment of uncomplicated cutaneous leishmaniasis cases with local thermotherapy and cond The project will contribute towards the World Health Organization Neglected Tropical Diseases roadmap targets for the control and elimination of visceral leishmaniasis uct operational research to improve access to VL diagnosis and treatment.
selected resources
Barriers to access visceral leishmaniasis diagnosis and care among seasonal workers and farmers in Tigray, Ethiopia - Coulborn R - Video 2017 (EN)
Barriers to access visceral leishmaniasis diagnosis and care among seasonal workers and farmers in Tigray, Ethiopia - Coulborn R - Abstract 2017
Selected publications
Determinants of Visceral Leishmaniasis: A Case-Control Study in Gedaref State, Sudan.
Barriers to access to visceral leishmaniasis diagnosis and care among seasonal mobile workers in Western Tigray, Northern Ethiopia: A qualitative study.
Clinical epidemiology, diagnosis and treatment of visceral leishmaniasis in the Pokot endemic area of Uganda and Kenya.
Diagnostic accuracy of two rK39 antigen-based dipsticks and the formol gel test for rapid diagnosis of visceral leishmaniasis in northeastern Uganda.
Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980-2004.
References
1 https://www.who.int/fr/news-room/fact-sheets/detail/leishmaniasis
2 Eco-epidemiology of visceral leishmaniasis in Ethiopia Endalamaw Gadisa, Teshome Tsegaw, Adugna Abera, Dia-Eldin Elnaiem, Margriet den Boer, Abraham Aseffa, Alvar Jorge Parasit Vectors . 2015 Jul 19;8:381. doi: 10.1186/s13071-015-0987-y.
3 Tsegaw T, Gadisa E, Seid A, Abera A, Teshome A, Mulugeta A, Herrero M, Argaw D, Jorge A, Aseffa A (2013) Identification of environmental parameters and risk mapping of visceral leishmaniasis in Ethiopia by using geographical information systems and a statistical approach. Geospat Health 7: 299-308
4 Bush JT, Wasunna M, Alves F, Alvar J, Olliaro PL, Otieno M, Sibley CH, Strub WN, Guerin PJ (2017) Systematic review of clinical trials assessing the therapeutic efficacy of visceral leishmaniasis treatments: A first step to assess the feasibility of establishing an individual patient data sharing platform. PLoS Negl Trop Dis 11: e0005781.
5 WHO (2010) Control of the Leishmaniases: Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22-26 March 2010.