Pneumococcal Disease: Fractional Dose PCV
PCV
In 2015, there were over 9 million cases of pneumococcal disease globally, with 2.4 million cases in Africa alone. 318,000 deaths were attributed to the disease, representing 10% of all deaths in children under 5 years of age, primarily in sub-Saharan Africa.
Our study, “Determining whether mass campaigns with fractional dose PCV10 would accelerate herd protection against pneumococcal transmission in Sub-Saharan Africa” explores the utility of mass campaigns of pneumococcal conjugate vaccine (PCV) among children in Niger. The PCV used in the trial is called Pneumosil® and protects against 10 of the most common serotypes of Pneumococcus. Three doses of PCV are usually given in routine infant immunization programs, but this trial will administer a single dose of PCV in mass campaigns among older children. Children in some villages will receive a full dose of PCV10, and others will receive a 20% dose, or “fractional dose”. The results of the trial are expected to provide evidence of the population-level direct and indirect impact of these campaigns in older children. The trial results will be further complemented by mathematical modelling, to inform the policy debate regarding PCV dosing schedules in different contexts. This trial and the modelling exercises that follow, would allow for larger scale evaluation of fractional dose PCV strategies in different contexts.
The study will be implemented by a consortium of partners, including Epicentre (the project lead), Université Abdou Moumouni, African Research Collaboration for Health Limited (KWTRP-KEMRI), and the London School of Hygiene and Tropical Medicine. This project is part of the EDCTP2 programme supported by the European Union (grant number RIA2020I-3282-fPCV).
Objectives
To evaluate
- whether a mass vaccination campaign targeting children approximately 1-9 years of age with a single full dose of PCV is superior to the absence of vaccine in reducing the proportion of children who are carriers of vaccine-serotype pneumococci (VT) at 6 months post-campaign,
- and subsequently to evaluate if a campaign using a single 20% dose of PCV is non-inferior to the full dose campaign.
Methodology
A Phase IV, 3-arm, observer-blinded, cluster-randomized controlled trial will be implemented in rural villages of the Madarounfa District of Niger.
A baseline survey will describe prevalence of nasopharyngeal carriage of pneumococci among children aged 1-9 years in each of the 63 villages included in the trial. The villages will be randomized to receive a campaign with full dose PCV, fractional dose PCV or no vaccination campaign (control arm) in a 3:3:1 allocation ratio. A second survey several months after the campaigns will assess nasopharyngeal carriage among children aged 1-9 years, and the results will be compared to the baseline.
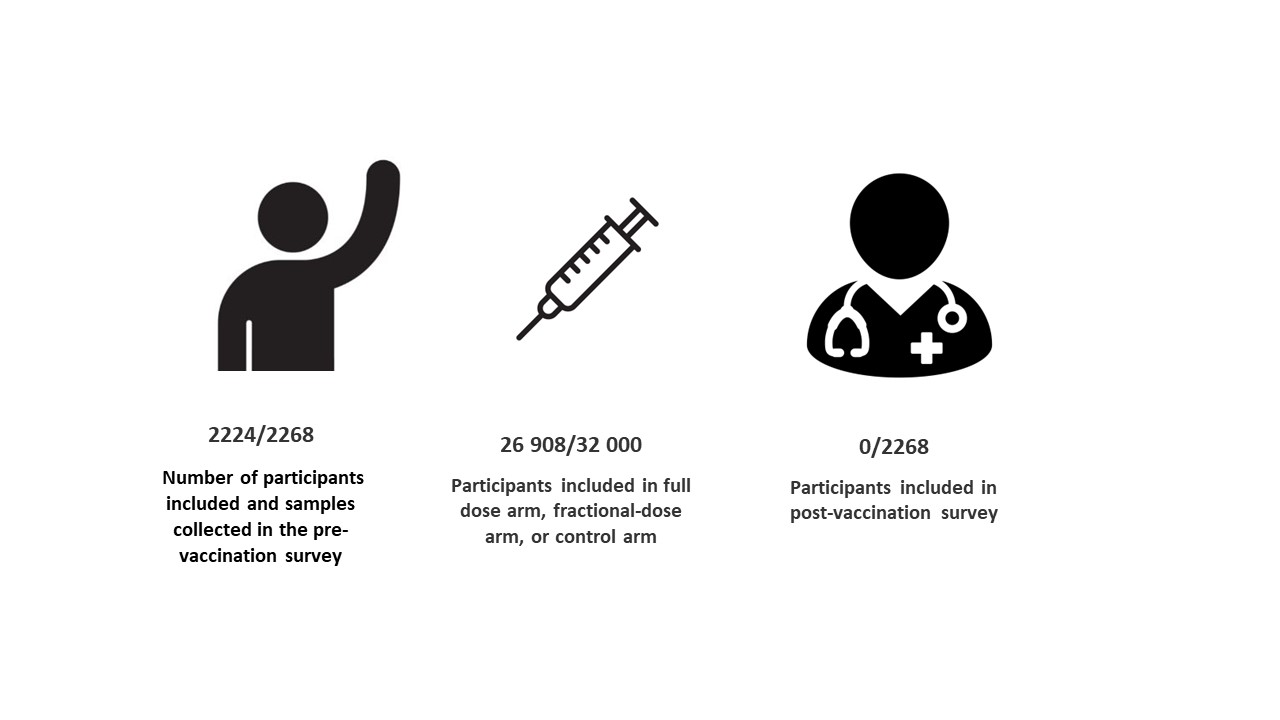
The project in numbers
On December 12, 2022 began the post-vaccination survey that will last 3 months.
Fractional Dose PCV Workpackage
Clinical Trial Implementation
Lead : Epicentre
Objective
- Implement and ensure the proper execution of the clinical trial
The Trial will be conducted to test the non-inferiority of a fractional dose of PCV administered between 1 and 9 years of age compared to a full dose of PCV administered between 1 and 9 years of age. The trial will be conducted at Epicentre’s Research Site in Maradi, Niger, and include 63 villages from the nearby Madarounfa District.
The vaccines used in the study will be provided by Serum Institute of India Private, Limited.
The primary outcome of this trial will be an assessment of the community-level prevalence of vaccine-serotype pneumococci before and after mass vaccination campaigns. The nasopharyngeal samples collected in the surveys will be analyzed by African Research Collaboration for Health Limited, in Kilifi, Kenya.
Project Management
Lead : Epicentre
Objective
- Timely and effective execution of the project
The project will be conducted over 18 months, including the clinical trial, laboratory and data analysis of the trial, and a training and professional development program. A Trial Steering Committee (TSC), composed of experts in pneumococcal disease prevention and epidemiology, as well as data management, ethics, and knowledge of the local community has been formed. A Data Safety and Monitoring Board (DSMB) has been established and regularly reviews trial data.
Data Management and Analysis
Lead : Epicentre
Objective
- Protection and management of data quality, as well as the robust analysis of data from WP1 and WP4
Epicentre is responsible for data management, including but not limited to the data management plan, the design and implementation of the clinical database management system using REDCap software system (www.project-redcap.org) and the delivery of a final study database. The data management plan is designed to set out the procedures of data management ensuring data quality and security. Data sharing, confidentiality, and security procedures are priorities of the consortium.
Laboratory Analysis
Lead : African Research Collaboration for Health Limited (KWTRP-KEMRI)
Objectives
- To ensure availability of material needed for collection, transport and storage of nasopharyngeal samples at the Niger site
- To test for the presence of Streptococcus pneumoniae in nasopharyngeal samples, identify pneumococcal serotypes and report results at regular intervals
- To assure the quality of sample collection
The KEMRI laboratory is a world leader in evaluations of Streptococcus pneumoniae, and will test all samples taken during this study. Confirmatory pathogen and serotype testing will be done on 10% of culture positive samples by PCR. An important aspect of the consortium is that their laboratory expertise will be shared with partners in Niger (see Training and Capacity Building below).
Training and Capacity Building
Lead : African Research Collaboration for Health Limited (KWTRP-KEMRI)
Objective
- To develop the capacity of Niger site staff to assess nasopharyngeal carriage of pneumococci through a tailored peer-assisted learning program
One of the aims of the consortium is to develop the capacity of Niger site staff to assess nasopharyngeal carriage of pneumococci in accordance with WHO standards through a tailored peer-assisted learning program. This program will be led and developed by African Research Collaboration for Health, Limited (KWTRP-KEMRI). Focus areas for the learning program will include: nasopharyngeal sample collection, transport and processing; identification of Streptococcus Pneumoniae by culture; serotyping; quality assessment; and laboratory information systems.
Cost-effectiveness and modelling
Lead : African Research Collaboration for Health Limited (KWTRP-KEMRI)
Objectives
- To model the impact of a mass vaccination campaign with fractional dose PCV on VT transmission
- To assess the cost-effectiveness of a mass vaccination campaign with fractional dose PCV in Niger and Kenya
Once the results of the main trial are available, age-structured, dynamic, deterministic models of pneumococcal transmission will be constructed to estimate the impact of mass fractional dose PCV campaigns on VT carriage in distinct programmatic settings (Niger and Kenya). The models will use data from social contact matrices and up to date vaccine coverage estimates from existing literature. Based on the modelled epidemiological impact of PCV-10 mass campaigns, with both full and fractional doses, a cost-effectiveness analysis to estimate the incremental cost-effectiveness ratio of routine PCV-10 mass campaigns, at different intervals and in the two different settings, will be performed.
Policy Uptake and Access
Lead : Université Abdou Moumouni (UAM)
Objectives
- To describe facilitators and barriers to implementing mass campaigns of fractional dose PCV among parents and caregivers, healthcare workers, national and international stakeholders
- To generate insights and recommendations on how a potential future fractional dose PCV mass campaign could be successfully, planned, communicated, delivered and integrated into national immunization programs
A literature review of published articles and available reports from countries that have introduced government-led mass vaccination campaigns in the 1-9 year age group will be conducted. The findings of this literature review will influence interview and focus group discussion topic guides. Focus group discussions and key informant interviews will be conducted across stakeholders and the community in order to inform the future planning of a largescale immunization campaign. Data will be collated and a set of draft ‘take home messages’ will be presented to key national and international stakeholders. Recommendations will be synthesized into a series of short 2-page policy briefs on that will include the following themes: planning a fractional dose PCV campaign, social mobilization, vaccine delivery, measuring coverage/ evaluating, fractional dose vaccine management and dispensing, projected impact and cost effectiveness, and sustainability. Each policy brief will include the implications of the findings and recommendations. We will in particular focus on examining what is different for a PCV campaign compared to other vaccines (e.g. communication and messaging), rather than what is already addressed within existing recommendations for new vaccine introduction (such as waste management).
Dissemination and communication
Lead : London School of Hygiene and Tropical Medicine (LSHTM)
Objective
- To use creative mechanisms to disseminate the findings of the project to national and international stakeholders
The final work package will conduct the critical task of disseminating our findings widely and effectively. This will include key stakeholders both within the local community and international scientific networks.
Funding Institution
KEMRI PCV webpage
Find out more








