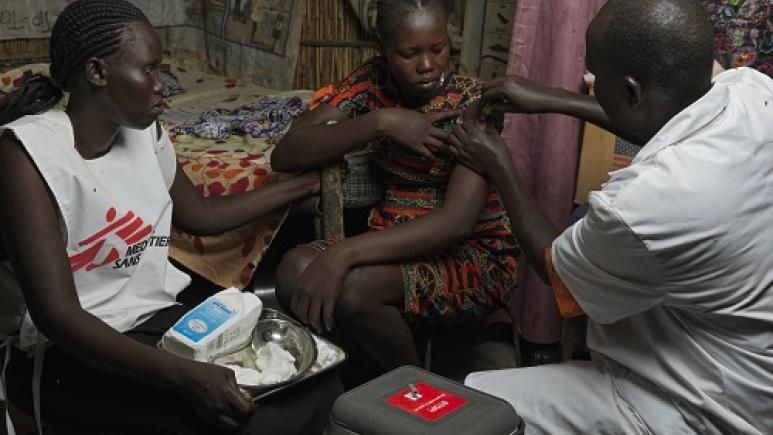
First evaluation of a mass vaccination campaign against hepatitis E
The most common cause of acute viral hepatitis, hepatitis E causes around 20 million infections and 44,000 deaths a year (1), mainly in the most vulnerable populations. The virus that causes hepatitis E is transmitted through faecal contamination of food and water. A lack of access to drinking water, inadequate water sanitation and poor hygiene, as is often the case in camps for displaced persons, create ideal conditions for the outbreak of large-scale epidemics. This is what has been observed in the Bentiu camp in the state of Unity in South Sudan. Exposed to outbreaks of hepatitis E since 2015, this camp, which is home to around 112,000 people, has seen the situation worsen in 2021 as a result of extreme flooding and the influx of displaced people.
To contain the epidemic, the South Sudanese Ministry of Health has asked MSF to carry out a vaccination campaign in the camp in 2022. Faced with the difficulty of implementing water and sanitation solutions in this context, this is the only "weapon" available, as there is no specific treatment for hepatitis E, which has a mortality rate of over 25% in pregnant women and also increases the risk of spontaneous abortions and stillbirths.
The only vaccine available against hepatitis E, Hecolin©, has been shown in clinical trials to be effective in preventing the disease. The World Health Organisation (WHO) has been recommending its use since 2015 to limit or prevent hepatitis E epidemics, as well as to mitigate the consequences in high-risk groups such as pregnant women. However, until now, it has only been administered on an individual basis in Asia, where it is authorized and used to vaccinate travelers. The vaccination campaign in Bentiu was the first in the world to use this vaccine in the context of an epidemic.
Initial assessment in an epidemic situation
In order to document and complete the information on this vaccine, Epicentre has collected data on vaccine coverage and acceptance and is currently carrying out analyses on the vaccine's efficacy and safety in real conditions.
"Across all three vaccination rounds, a total 39,764 people received the first dose, 26,110 received the second dose, and 14,293 received the full 3 dose schedule”, explains Robin Nesbitt, epidemiologist at Epicentre.
At the end of the 3rd vaccination round, interviews were conducted with a representative sample of 1,599 people eligible for vaccination. The results showed that 86% of the population eligible for the vaccine had received at least one dose, 73% at least two doses and 58% all 3 doses. There were no significant differences in vaccination coverage according to sex, age or place of residence in the camp.
Little reluctance to vaccinate and few side effects
60% of people who have not been vaccinated give as their reason that they were absent or unavailable at the time of the vaccination campaign. Fear of needles or the side effects of the vaccine were rarely mentioned.
"Very little reluctance towards the vaccine was noted, which can partly be explained by the fact that people know about hepatitis E and its consequences following the hepatitis E epidemics in the Bentiu IDP camp, but also other epidemics," notes Iza Ciglenecki, Operational Research Coordinator at MSF Switzerland. A qualitative study also revealed that all the participants in the discussion groups had personal experience of the disease, either because they were ill themselves or because they knew someone who was.”
Fewer than one in 8 people reported any side-effects within 72 hours of the first dose, and most often these were fever or pain at the injection site. Even so, this was more than the surveillance systems had recorded, which could be explained by the fact that these symptoms were of a moderate nature and did not justify the need for these people to seek medical attention.
Overall, the vaccination campaign has been well accepted by the population of the Bentiu camp, with few side-effects to be deplored. Epidemiologists are now analysing the data - still insufficiently documented to date - on the safety of Hecolin® in pregnant women.
A new mass vaccination campaign in isolated communities, this time in South Sudan
On the strength of this first encouraging vaccination campaign, at the end of 2023 MSF launched a new vaccination campaign in Fangak, one of the most remote areas of South Sudan, which turns into a gigantic swamp during the rainy season, and where a hepatitis E epidemic was officially declared by the local Ministry of Health in September 2023. Since the first case was detected in April 2023, there have been 20 deaths, mainly among pregnant women, as of 14 January. Given the risk to pregnant women, the campaign is targeting only women aged between 16 and 45. In addition to the logistical challenge involved, this campaign, which is taking place in a different context, will also be the subject of a new study by Epicentre: it will include a survey of vaccination coverage, passive surveillance of adverse events, a qualitative study of acceptance of the strategy of targeting women only, and a description of the epidemic using data collected from the line-list at the MSF hospital.
While these two studies help to fill in the gaps in the data available to date on the disease and the vaccine available, hepatitis E remains neglected by the research community. The resources allocated are far from commensurate with the challenges.
“Hepatitis E epidemics occur in low-income countries, among populations that are often isolated and affected by conflict", explains Etienne Gignoux, Director of Epicentre's Epidemiology and Training Department and involved in several hepatitis E projects. Research into treatments is virtually non-existent. Only one vaccine is available, and it has never been evaluated in epidemic conditions before 2022. There is an urgent need to develop reactive and preventive vaccine strategies for those most at risk and better treatments, but also to improve our knowledge of this disease and its impact".
The WHO's prequalification of the vaccine will not solve all the problems. For the moment, the vaccine is not authorized for people under the age of 16. As Robin Nesbitt points out, "We have seen in Bentiu that many children develop severe forms of the disease". So there are still many unknowns about this disease. Recognition of hepatitis E as a neglected tropical disease could " help break the cycle of neglect by accelerating advances in research and public health action and elevating epidemic hepatitis E in the global health agenda", as Iza Ciglenecki and other authors explained in an article published in Plos Neglected tropical disease in 2019 (2).
- https://www.who.int/news-room/fact-sheets/detail/hepatitis-e#:~:text=Key%20facts,symptomatic%20cases%20of%20hepatitis%20E.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6657817/