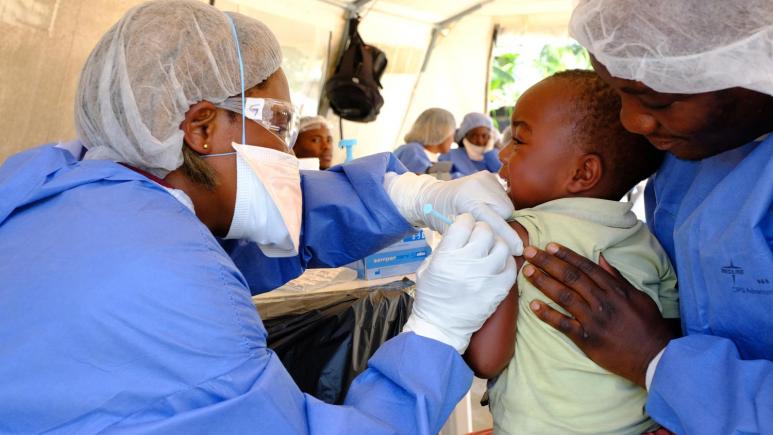
Ministry of Health in DRC approves Janssen Ebola vaccine for use
On 20 September, the DRC officially announced the use of a second vaccine to complement the current rVSV ring vaccination strategy. The vaccine, a two-dose vaccine regimen developed by Janssen pharmaceuticals, will be implemented in a protocol developed by l’Institut Nationale de Recherche Biomedical in conjunction with the London School of Hygiene and Tropical Medicine, Epicentre, MSF, and other partners.
The study in a nutshell
The current Ebola outbreak in North Kivu and Ituri provinces of the DRC has been raging since 1 August 2018, with over 3000 confirmed cases and 2000 confirmed deaths. Currently, the Merck rVSV-ZEBOV-GP vaccine is being implemented in a ring vaccination strategy. Upon recommendations of the Strategic Advisory Group of Experts SAGE and by request of DRC authorities, alternative vaccines were explored to complement the current Ebola epidemic control strategies.
The Janssen Ebola vaccine is administered in two doses, the first with the Ad26.ZEBOV vaccine, and the second 56 days later (-14 days; +28 days) with the MVA-BN-Filo vaccine. The study aims to vaccinate participants throughout North Kivu and Ituri provinces, with a primary objective of determining vaccine effectiveness. This study will additionally aim to answer secondary objectives regarding safety, coverage, and community perceptions of Ebola vaccination. Results and information derived from these objectives can inform further Ebola epidemic response as well as lead to finally having a licensed vaccine for Ebola.