Malaria: vaccines, hope and many challenges
In 2023, malaria affected 263 million people and caused almost 600,000 deaths (1). Africa remains the most affected region, accounting for 94% of cases and 95% of deaths. Among the victims, 76% were children under the age of five. Every hour, 50 children under the age of 5 die from malaria in Africa.
Effective vaccines, but a logistical challenge
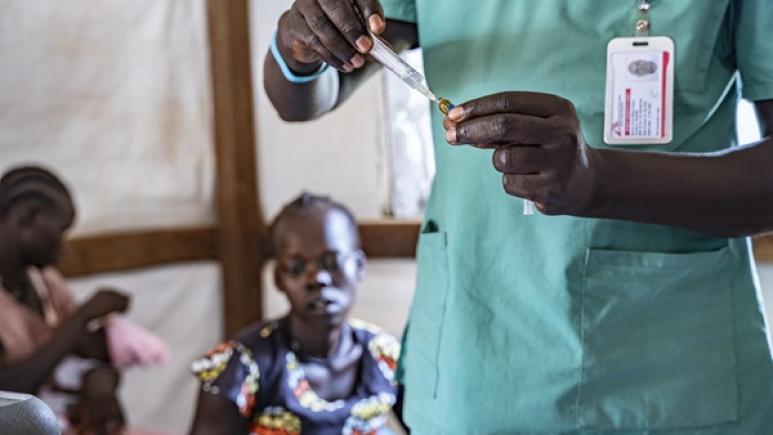
Despite initial global progress in prevention and treatment, malaria eradication remains a distant goal as this progress has stalled, and sadly in some countries cases and deaths are on the rise . In this context, the arrival of the RTS,S/AS01 and R21/Matrix-M™ vaccines raises immense hope, particularly for young children. As proof, in a Phase III clinical trial carried out on 4,800 children in Kenya, Tanzania, Burkina Faso and Mali, the R21/Matrix-M™ vaccine reduced the number of cases (detected by rapid diagnostic test(2)) by 75% in areas of seasonal transmission and by 68% in regions where malaria transmission occurs year-round.
As a result, WHO now recommends the use of RTS,S/AS01 and R21/Matrix-M™ vaccines to protect children living in areas with moderate to high transmission. But deploying these vaccines on a large scale remains a challenge.
Malaria vaccine administration: what strategies for optimal protection?
By 2024, more than 20 African countries had integrated malaria vaccines into their routine immunization programs (EPI), which aim to protect children aged 12 to 59 months against some twenty diseases. The initial 3 doses are administered from 5 months of age, according to an age-based schedule. However, this strategy may not be the most suitable for a number of reasons.
“RTS,S/AS01 and R21/Matrix-M™ require four doses: three initial ones one month apart, followed by a booster between 6 months and one year later, explains Pr Halidou Tinto, Regional Director of the Institut de Recherche en Sciences de la Santé in Nanoro, Burkina Faso. However, this schedule is not always aligned with routine EPI vaccinations, which complicates their integration."
What's more, in areas of seasonal transmission, the protection offered by the vaccine is limited in time, so a schedule based solely on age, like that of the EPI, does not guarantee optimal efficacy during the rainy season, a period of high transmission.
In a context of limited resources, optimizing vaccine deployment is essential. One solution being considered is to combine vaccination with Seasonal Malaria Chemoprevention (SMC), a strategy that has already been used for more than ten years in several African countries with seasonal malaria transmission. SMC involves giving children aged 3 to 59 months preventive treatment (sulfadoxine-pyrimethamine and amodiaquine) every month during the rainy season, through community-based interventions.
“In areas where malaria is seasonal, an annual campaign combining vaccination and SMC during the high transmission season could be an effective approach,” explains Valérie Briand, director of Epicentre's research department.
A clinical study conducted with RTS,S/AS01 has also shown that this combination reduces cases of uncomplicated and severe malaria by 60 to 70% compared to either intervention alone.
Combining vaccination and chemoprevention: a promising strategy
To test this approach, In collaboration with national malaria control programs and the immunization divisions of the countries concerned, Epicentre is launching two clinical trials to compare an R21/Matrix-M™ vaccination strategy integrated into SMC with an age-based strategy (integrated into the EPI) in areas with high seasonal transmission. The first is taking place in Chad through a collaboration with the Ministry of Health and funded by MSF and Expertise France. The second is taking place in partnership with health authorities in Burkina Faso and Mali as part of the IMVACS (Integrating Malaria Vaccine with Seasonal malaria chemoprevention in West Africa) consortium, via the EDCTP3 program supported by the European Union.
The objective is to evaluate the effectiveness of combining SMC with R21/Matrix-M™ vaccination, as well as vaccination coverage, adherence to the vaccination schedule, and the impact on other preventive measures. One part of the study will also document the acceptability, operational feasibility, and implementation of these two strategies—integrated into the EPI and SMC—as well as their cost and cost-effectiveness.
“Combining R21 vaccination with existing monthly SMC campaigns could lead to better vaccination coverage for the essential third dose and the fourth (booster) dose, optimizing protection for children, particularly at the tail end of the long rainy season, when we generally still see a high burden of cases after SMC activities end. In addition, the simultaneous provision of these two proven interventions, which are both already separately recommended by WHO in the same population, is expected not only to have a synergistic effect, but may even demonstrate a greater cost-effectiveness overall. This will be an important consideration, particularly in the unstable future funding landscape” emphasizes Saschveen Singh, Tropical Infectious Diseases Advisor for MSF France.
“If this approach proves successful, it could transform the fight against malaria by making vaccination more accessible to the most vulnerable populations in areas where transmission is seasonal and where malaria peaks lead to high morbidity and mortality,” concludes Prof. Halidou Tinto.
This strategy would prioritize those who, due to limited or delayed access to healthcare, remain the primary victims of the deadly disease.
(1) https://www.who.int/fr/news-room/fact-sheets/detail/malaria
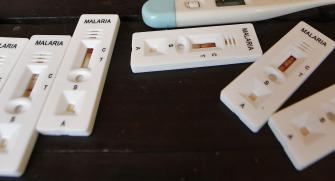
(2) Malaria rapid diagnostic tests (RDTs) assist in the diagnosis of malaria by providing evidence of the presence of malaria parasites in human blood.
© Paula Casado Aguirregabiria